Today is Clinical Trials Day – celebrated to bring more attention to public health and also to recognize the contribution of the patients and healthcare professionals who make clinical research possible. At PatientsLikeMe, it’s members who are changing the way clinical trials are designed.
Bringing the patient voice to clinical trials has long been part of the PatientsLikeMe mission. Jeremy Gilbert, Vice President, PLM Health and Paul Wicks, Ph.D., Vice President, Innovation, sat down with us last year to talk about the importance of putting patients at the center of drug discovery and development. Check out their Q&A here. Recently, Paul Wicks touched on the purpose behind the latest PatientsLikeMe study on clinical trial design involving the patient perspective, and why organizations need to work on improving their trial process:
“As researchers we know that clinical trials are the best tool we have for identifying new, safe, effective treatments. Patients know this, too, and they’re motivated to take part. But what this research tells us is that actually participating in a trial is not a fun experience; about as much fun as dealing with the worst airlines, banks, or utility companies, and we all know how that can be. This is a call to action to trial designers and sponsors to step up their game and understand that while patients volunteer out of altruism, a clinical trial still has to fit into their daily life and should create as little burden as possible if we want people to enroll and see it through to the end.”
-Paul Wicks
4,718 PatientsLikeMe members took part in the survey, and below is just a snippet of what they had to say. The complete findings of this study have also recently been published – take a look!
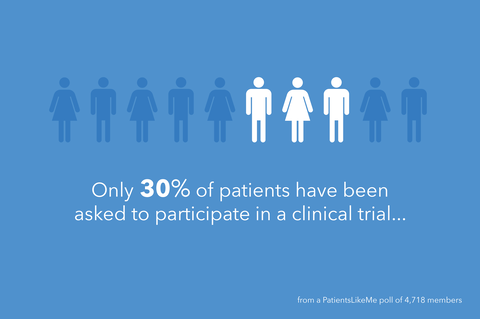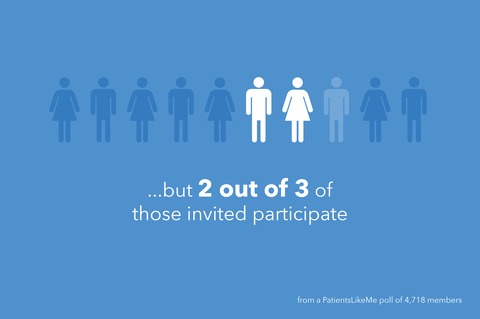
How do patients learn about clinical trials?
59% of those who responded said they learned about a trial from their health team, while 24% said they learned via the web. For those who participated in past trials, the first person to suggest they participate was a doctor (43%) or another healthcare provider (19%), and 80% of respondents said they took part in the trial based on their own desire to.

A key takeaway from the study:
Most people are still finding out about trials through their care teams or providers, but when it comes to making a decision to take part, it’s their own desire that motivates them.
Paul Wicks weighed in saying, “We think patients are interested in participating in research in general because of altruism, that they choose to enroll in a particular trial because of its objectives, and that they stay enrolled because of their relationship with trial staff and the level of burden the study incurs on their daily lives.”

What are patients’ impressions of clinical trials?
Of those who responded, 55% were very or extremely satisfied, and 51% would tell other patients about the trial.
Jeremy Gilbert touched on the issue of patients providing feedback following a trial, “We’re starting to see another gap now, which is that companies have no way of soliciting feedback from patients as they participate in a trial, to find out what patients think of real trials. This is a surprise, because given most of us are consumers, we’re used to being able to give feedback about a product or our experience at any time.”

9% of those who answered the survey considered dropping out of their trial — side effects and worsening of overall health after the trial were the main reasons. Following the conclusion of a trial only 38% of patients recall being told about the results.

To find out more about clinical trials and how to get involved, visit the PatientsLikeMe clinical trial finder tool. Find a trial that’s right for you, search by location, phase, intervention type and more.
Thank you to all who participated and shared their experiences to help bring the patient perspective into improving clinical trials.
 Share this post on Twitter and help spread the word.
Share this post on Twitter and help spread the word.

You touch an extremely important topic. To leverage the outcome of clinical trials for participating patients a role should be defined. And – a feedback to participating patients should be mandatory.
Not all trials involve placebo. I have been in 3 trials with no placebo. II did receive a placebo my treatment would not be compromised. More patients should participate to move research forward.
Jenny Hack
I would like to know about trials
We are snow birds, and my Neurologist says it makes me ineligible for clinical trials, is this true for all clinical trials? (Parkinson’s)
Hi Laurie,
Being a snowbird may not be a blanket exclusion to participate in a clinical trial – it may be more a factor of the travel and time away that could affect one’s ability to meet all of the study obligations and continue to be evaluated and assessed at the study site at the appropriate time points. This depends on the trial requirements and the timing of clinic visits and how traveling away from the trial’s primary location throughout the year might impact that. It may not be a fair blanket statement to say that all snowbirds are ineligible for clinical trials, but it would likely complicate participation in a number of studies if travel and time away affects clinic visits. A conversation that might perhaps be worth pursuing with your Neurologist is around any potential shorter trials that wouldn’t conflict with your travels.
Dear PatientLikeMe,
I completely agree that being a snowbird does not make a person ineligible for clinical trials. It is shocking that a physician would say this to a patient. I have worked in the pharmaceutical sector for the past 12 years, and it has been my experience that most clinical trials have so poor patient recruitment numbers, that they are willing to be very accommodating for patients. In some instances, paying for patients to travel or conducting visits at the patients home.
Sadly if you research the topic, you will find that less than 10% of doctors in the US are willing to suggest clinical trials to patients, despite the fact that in many cases the current standard of care is ineffective. Please advise your readers to seek out clinical trial information from an unbiased source, like clinical trials.gov, to find a trial that could help them.
Recent family issues have brought this topic close to my heart, and I would be willing to help anyone who needs help to find a clinical trial that they may be eligible for.